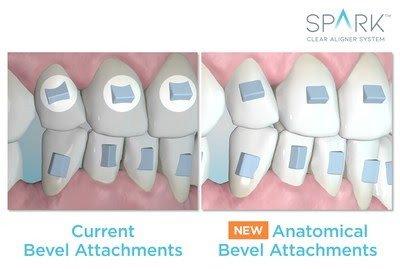
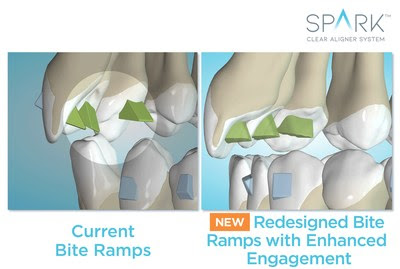
New additions reflect the diverse, global perspective of the business school community
RESTON, Va., June 23, 2021 (GLOBE NEWSWIRE) — The Graduate Management Admission Council™ (GMAC™), a global association of leading graduate business schools, today announced the addition of four new members to its board of directors. Katy Montgomery, Associate Dean, Degree Programmes, INSEAD; François Ortalo-Magné, Dean, London Business School; and Giuseppe Soda, Dean, SDA Bocconi School of Management, Bocconi University, will begin their terms on July 1. In addition, Yuan Ding, Vice President and Dean of China Europe International Business School (CEIBS), was appointed as a board director in January this year to fill the seat vacated by Enase Okonedo of the Pan-Atlantic University.
“GMAC’s new board of directors represent leading business schools with campuses located in 10 countries across Europe, Asia, North America, Africa, and the Middle East,” said Sangeet Chowfla, president and CEO of GMAC. “As student mobility returns and regains in the post-pandemic world, I look forward to working alongside this diverse group of leaders ― and the rest of the GMAC board ― to continue to advance GMAC’s vision to ensure that all talented individuals can benefit from the best business education for them.”
New GMAC Board Members
Yuan Ding, Vice President and Dean, Cathay Capital Chair in Accounting, CEIBS
Yuan Ding is Vice President and Dean and the Cathay Capital Chair Professor in Accounting at CEIBS, where he has been honored three times with the CEIBS Teaching Excellence Award. Prior to joining CEIBS, he was a tenured faculty member of the HEC School of Management, Paris, France. He is a member of the European Accounting Association, French Accounting Association and American Accounting Association. He holds a PhD in Accounting from the Institute of Enterprises Administration at the University Montesquieu Bordeaux IV, France, as well as a Master’s in Enterprises Administration from the University of Poitiers, France. Ding is the author of multiple books on financial reporting and his research appears in leading academic journals.
Katy Montgomery, Associate Dean, Degree Programmes, INSEAD
As the INSEAD Associate Dean of Degree Programmes, Montgomery is responsible for the commercial leadership of the INSEAD Degree Programme portfolio across four campuses: Fontainebleau, Singapore, Abu Dhabi, and San Francisco. Her functional responsibilities include strategy, marketing, sales, admissions, financial aid and scholarships, programme operations, student life, psychological services, and career services. Prior to joining INSEAD, she served as Associate Dean of Student Development at Johns Hopkins Carey Business School. Montgomery holds a degree in Political Science from Loyola University New Orleans and a Juris Doctor degree from Georgetown University Law Center.
François Ortalo-Magné, Dean, London Business School
François Ortalo-Magné is the ninth Dean of London Business School (LBS), a position he has held since August 2017. He is leading a strategy focused on (1) academic research and its impact, (2) learning innovations and alumni engagement and (3) inclusion, striving for gender parity and greater socio-economic and ethnic diversity. Since taking up the role, Ortalo-Magné has led the relaunch of the LBS brand, the growth of degree programmes and a significant increase in philanthropic support for scholarships. His research on the economics of land and housing markets has been published in leading academic journals. He has built on his research and leadership experiences to advise a broad range of private, governmental and multi-lateral organisations and share his insights in leading media outlets and at conferences around the world. Prior to his appointment, Ortalo-Magné was the Albert O. Nicholas Dean and Robert E. Wangard Professor of Real Estate at the Wisconsin School of Business. His first academic appointment was at the London School of Economics.
Giuseppe Soda, Dean, SDA Bocconi School of Management, Bocconi University
Giuseppe “Beppe” Soda is the Dean of SDA Bocconi School of Management and Full Professor of Organization Theory and Network Analysis at Bocconi University. Before becoming Dean in 2016, his roles have included serving as the Associate Dean for Research (2007-2013), Director of the Department of Management and Technology (2013-2016) and Head of Organization and HRM Department (2001-2006). He is also serving EFMD as member of the EQUIS Accreditation Board. Soda’s research investigates the performance consequences of the interplay between organizational architectures and organizational networks and his work has been published in top academic management journals.
Besides the aforementioned newly elected board members, Martin Boehm, Professor of Marketing and former Dean of IE Business School and soon the new Rector of EBS Universität für Wirtschaft und Recht, and Themin Suwardy, Dean of Postgraduate Professional Programmes, Singapore Management University, were re-elected for a second term.
GMAC also recognizes its outgoing board members, Leila Guerra, Vice Dean (Education) of Imperial College Business School, and Peter Tufano, Peter Moores Dean and Professor of Finance of Saïd Business School, University of Oxford. GMAC thanks them for their service in the past nearly four years to our organization and contributions to the graduate management education community.
About GMAC™
The Graduate Management Admission Council™ (GMAC™) is a mission-driven association of leading graduate business schools worldwide. Founded in 1953, GMAC creates solutions and experiences that enable business schools and candidates to better discover, evaluate, and connect with each other.
GMAC provides world-class research, industry conferences, recruiting tools, and assessments for the graduate management education industry, as well as tools, resources, events, and services that help guide candidates through their higher education journey. Owned and administered by GMAC, the Graduate Management Admission Test™ (GMAT™) exam is the most widely used graduate business school assessment.
GMAC also owns and administers the NMAT by GMAC™ (NMAT™) exam and the Executive Assessment (EA). More than 7 million candidates on their business master’s or MBA journey visited GMAC’s mba.com last year to explore business school options, prepare and register for exams, and get advice on the admissions process. BusinessBecause and The MBA Tour are subsidiaries of GMAC, a global organization with offices in China, India, the United Kingdom, and the United States.
To learn more about our work, please visit www.gmac.com.
Media Contact:
Teresa Hsu
Sr. Manager, Media Relations
202-390-4180 (mobile)
thsu@gmac.com




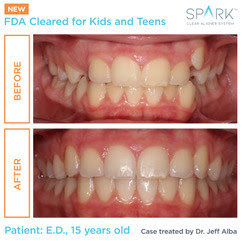
 “My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”
“My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”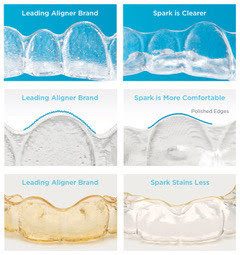 “My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.”
“My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.” “Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”
“Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”